News
Medical device touch screen standards
Medical device touchscreen standards
As artificial intelligence continues to enter our lives, more and more electronic equipment has also been added to intelligent technological elements, including the development trend of the Internet, which has brought technological medical terminals to the forefront.
Are there any requirements for medical touchscreens used on medical equipment? The more common requirements are transmittance, touch with gloves, waterproofing, sensitivity, etc. In fact, there is also a testing standard for temperature and EMC. In fact, this is a Touch screen and there are no testing requirements. The main thing is the display screen and installation. It is a testing standard for all aspects of the complete machine.
In the field of medical devices, touchscreen standards cover the following aspects:
1. Safety standards: Medical device Touch screens need to comply with relevant safety standards to ensure their safety and reliability in the medical environment. For example, the IEC 60601 series of standards issued by the International Electrotechnical Commission (IEC) stipulates the safety requirements for medical electrical equipment, including safety requirements for touch screens.
2. Electromagnetic compatibility (EMC) standards: Medical device Touch screens need to meet electromagnetic compatibility standards to ensure that they do not cause electromagnetic interference to other equipment or patients in the medical environment. For example, the European standard EN 60601-1-2 specifies electromagnetic compatibility requirements for medical electrical equipment.
3. Antibacterial standards: Medical device Touch screens often need to have antibacterial capabilities to prevent the spread of bacteria and viruses on the touch screen surface. Relevant antimicrobial standards can vary based on specific regions and applications, but common standards include ISO 22196 and JIS Z 2801, among others.
4. Medical interface standards: Medical device Touch screens need to provide an interface that is easy for medical professionals to use to support clinical diagnosis and treatment operations. For example, the user interface of a touch screen should be ergonomic and easy to operate and understand.
5. Durability and reliability of Touch screens: Medical device Touch screens need to be durable and reliable, and can maintain good performance under high-frequency use and harsh conditions. This includes touch screen material selection, water and dust resistance, mechanical strength and reliable touch performance.
The following are some common standards related to medical device touch screens:
IEC 60601-1: This is one of the series of safety standards for medical electrical equipment developed by the International Electrotechnical Commission (IEC). As part of medical equipment, touch screens need to comply with this standard to ensure the safety and electrical performance of the equipment.
IEC 60601-1-2: This is the electromagnetic compatibility standard for medical electrical equipment. For medical device touch screens, it needs to comply with this standard to ensure normal operation and interference-free performance in the electromagnetic environment.
ISO 14971: This is the international standard for risk management of medical devices and also applies to touchscreen systems. The standard requires manufacturers to conduct risk assessments and controls to ensure patient and operator safety when using touch screens.
ISO 9241-400: This is an ergonomics standard that includes touchscreen design and human-machine interface requirements. For medical device touch screens, it needs to meet this standard to provide a convenient, easy-to-use and user-friendly operating interface.
FDA 510(k) certification: For medical device touchscreens sold in the United States, manufacturers need to pass FDA 510(k) certification to confirm that they comply with relevant regulations and standards to ensure that the product can be market-compliant.
Medical device Touch screen standards: It is important to note that specific standards and requirements may vary by region, application and type. Therefore, the standards for touch screens in the design and production of medical devices are specifications and requirements established for the design, manufacture and use of touch screens in medical equipment. The purpose of these standards is to ensure that the safety, reliability and performance of medical device touch screens comply with international and industry standards.
DINGTOUCH Touch screen production touch screen has more than 12 years of R&D and manufacturing experience, and engineers who have grown together for more than 5 years are designed to provide you with a suitable touch solution.
Do you know what other standards are for medical device touch screens?
You can discuss and chat together.
DINGTOUCH: Committed to continuous innovation and improvement of product quality to meet customers' high requirements and expectations.
DINGTOUCH is a manufacturer that provides high quality touch screen panels. Focus on the design, manufacturing and sales of touch screen panels, and are committed to providing customized solutions that satisfy customers.
DINGTOUCH: In the process of customizing touch screen panels, we focus on close cooperation and communication with customers. Understanding customers' needs and providing customized solutions will meet customers' individual needs. The company's products are favored by customers for their high quality and reliability, and provide them with the best touchscreen panel solutions.
At DINGTOUCH, we are the world's leading touchscreen manufacturer, helping businesses around the world take advantage of this exciting technology. For more information, please visit the home page now.
How to choose touch screen customization?
Find the DINGTOUCH technical team to achieve the success of your company's new project.
DINGTOUCH is a company specializing in the R&D and production of touch screen technology, headquartered in Shenzhen, China. As a professional touch screen supplier, DINGTOUCH is committed to providing high-quality, stable and reliable touch screen products to meet the diverse needs of customers. We continue to carry out technological innovation and product optimization to ensure that its touch screen products have good sensitivity, accuracy and durability.
In addition to the products themselves, we also focus on cooperation and communication with customers, and are committed to providing customized solutions and excellent after-sales services. Through continuous efforts to improve product quality and customer satisfaction, we have established a good reputation in the touch screen industry and won widespread market recognition.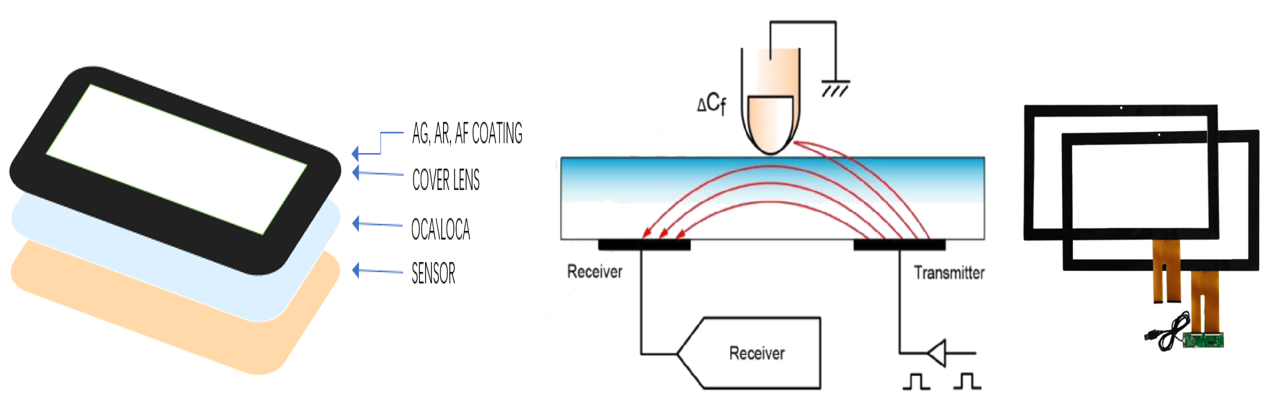
What DINGTOUCH can do:
• PCAP maximum size 65”
• Multi-touch (Touch screen can be customized to your needs.)
• Optical bonding service/air bonding
• LCD interface: HDMI/RGB/MIPI/LVDS/EDP, etc.
• PCAP interface: IIC/USB interface
• CTP can customize the cover glass surface treatment process AG (anti-glare), AR (anti-reflection), AF (anti-fingerprint), waterproof, and glove touch
• Supports 0.55 mm-12 mm coverslip touch.
• Support operating temperature: -40℃-90℃.
Dingtouch Industrial Capacitive Touch Screen Manufacturer
In conclusion, Dingtouch as a professional touch screen manufacturer with more than 10 years touch screen experience.We have many capacitive touch screen. Such as5 inch touch screen,7 inch touch screen,10.1inch touch screen,15 inch touch screen,15.6 inch touch screen,17 inch touch screen,18.5 inch touch screen,19 inch touch screen,21.5 inch touch screen,32 inch touch screen, However, we also welcome to customize your own touch screen . Contact our team today to learn what capacitive touch screen are best for our retail business needs.
Contact us NOW! sales@szdingtouch.com
CATEGORIES
CONTACT US
Contact: Dingtouch
Phone: +8615815536116
Tel: +8615815536116
Email: sales@szdingtouch.com
Add: Building A, Bailu Plaza, No. 48, Gonghe Industrial Road, Gongle Community, Xixiang Street, Baoan District, Shenzhen,China. 518126




 Dingtouch
Dingtouch